Select your destination
Continue to Radiometer Medical
βhCG
The biomarker of choice to aid in the early detection of pregnancy
-
 Fast results
Fast results
-
 Quantitative results
Quantitative results
-
 Early pregnancy detection
Early pregnancy detection
Pregnancy testing in the emergency department
Testing for pregnancy is common clinical practice when women are admitted to the emergency department (ED). Pregnancy is often the clinical cause of the symptoms at presentation, and can also be a risk factor for other serious disorders, including urinary tract infection and venous thromboembolism (VTE) [1,2].
When a woman presents with symptoms such as severe abdominal pain or swelling of the legs or has trauma requiring radiography, a fast and accurate pregnancy test can provide critical information for further treatment and management of the patient’s disorder
A quantitative blood test measuring beta human chorionic gonadotropin (ßhCG), performed at the point-of-care, can rapidly deliver accurate results for this purpose [3].
Immunoassay products and solutions

ßhCG - an effective biomarker for pregnancy
Most pregnancy tests measure ßhCG levels, which rise quickly in the first few weeks of pregnancy. ßhCG can be detected in blood and urine samples but is typically more concentrated in blood. Pregnancy blood tests can detect ßhCG about 11 days after conception, several days earlier than standard urine tests [6].
Pregnancy blood tests are also more sensitive than typical urine dipstick tests, providing quantitative results to support clinical evaluation [3].
Implications of pregnancy for clinical management
Pregnancy is also an indication to minimize unnecessary radiographic treatments, such as X-rays, which could potentially harm the developing fetus. General guidance recommends that a pregnancy test should be considered prior to radiography if a patient’s period is overdue [8].
ßhCG testing at the point of care accelerates ED turnaround time
A key reason for this reduction is that blood testing circumvents the need to wait for urine samples. Significant delays may occur if patients are not ready to provide urine or are unable to provide samples due to illness or injury.

Testing ßhCG on the Radiometer AQT90 FLEX
The AQT90 FLEX analyzer delivers lab-standard quantitative results on the ßhCG assay in less than 19 minutes, accelerating pregnancy diagnosis in the ED to support rapid clinical decision making.
The AQT90 FLEX immunoassay analyzer is a benchtop analyzer that brings rapid biomarker assessment capabilities right to the patient’s bedside.
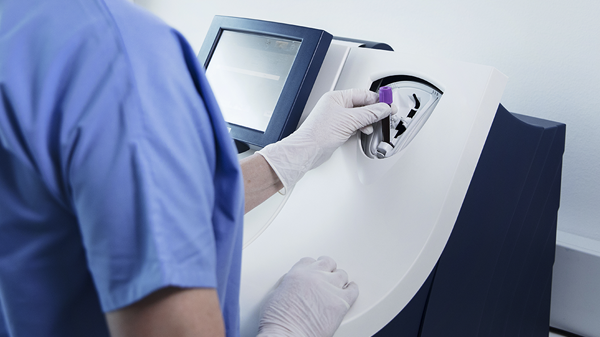
Its closed tube system makes ßhCG testing easy: the operator simply inserts the sample tube into the tube holder in the sample port and the analyzer performs all assay steps automatically. There is no need for sample preparation.
All necessary reagents are included in the test cartridges, which remain stable onboard the analyzer for 20 days ensuring maximum availability and uptime.
Quick facts on ßhCG assay on the AQT90 FLEX analyzer
- Specimen types: venous whole blood and plasma
- Sample tubes: fit most 13 × 75 mm standard tubes
- No blood exposure: closed system
- No sample or assay preparation
- High analytical performance
- No presence of hook effect
- Hemolysis, lipemia, icteria and biotin* show no interference with the assay.
*No interference was observed with biotin up to 2.6 mg/L (2,600 ng/mL).
ßhCG assay specifics
Pre-menopausal < 2 IU/L
Males < 0.5 IU/L
References
2. Tintinalli J, Thomas S, Ma J, et al. Emergency Medicine, A Comprehensive Study Guide, McGraw-Hill Education, Oct. 2019
3. Fromm C, Likourezos A, Haines L, et al. Substituting whole blood for urine in a bedside pregnancy test. J Emerg Med. 2012;43(3): 478–82.
4. Korevaar TIM, Steegers EAP, de Rijke EB, et al. Reference ranges and determinants of total hCG levels during pregnancy: the generation R study. Eur J Epidemiol. 2015; 30: 1057–66.
5. Castillo JC, Humaidan P, Bernabéu R. Pharmaceutical options for triggering of final oocyte maturation in ART. Biomed Research International. 2014; Art.ID. 580171.
6. Chard T., REVIEW: Pregnancy tests: a review. Human Reproduction. 1992; 7: 701–710.
7. Niebyl JR. Drug use in pregnancy and lactation. In: Pearlman MD, Tintinalli JE, eds. Emergency Care of the Woman. New York: McGraw Hill; 1998: 165–178.
8. British Institute of Radiology, Society and College of Radiographers and the Royal Society of Radiologists. A guide to understanding the implications of Ionising Radiation (Medical exposure). Regulations in diagnostic and interventional radiology. London; The Royal Society of Radiologists, 2015.
9. Gottlieb et al. Comparison of Urine and Whole Blood Pregnancy Testing. Western Journal of Emergency Medicine. 2016; 17:449–453
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.